Impact of Water Quality on Energy Consumption in PEM Water Electrolysis for Hydrogen Production
- 逸风 黄
- Sep 30, 2025
- 3 min read
Proton Exchange Membrane (PEM) electrolysis has become a mainstream method for hydrogen production due to its high efficiency, large current density, wide operating temperature range, and rapid response. While much research focuses on PEM stack demonstration, novel catalyst development, or membrane improvement, the optimization of the system and feedwater remains a critical challenge. This study investigates the impact of key water quality parameters—pH, Total Dissolved Solids (TDS), and conductivity—on the energy consumption of PEM electrolyzers to optimize the hydrogen production process. These interconnected parameters significantly influence electrolysis performance. The core principle of PEM electrolysis involves the electrochemical splitting of water into hydrogen and oxygen at the electrodes. As water is the primary reaction medium, its quality directly affects efficiency and energy consumption.
Key factors include:
pH can alter the oxygen evolution reaction potential, affecting energy use, but extreme pH values can cause membrane degradation.
Conductivity: Lower conductivity can reduce energy consumption, but excessively high values may damage the membrane and increase parasitic loads.
TDS, related to conductivity, can lead to scaling issues. The American Society for Testing and Materials (ASTM) recommends using Type I deionized water (TOC <50 ppb, resistivity >1 MΩ·cm, Na/Cl <5 µg/L). However, real-world water sources contain impurities, increasing purification costs. Studies indicate that zero TDS results in no hydrogen production, while high TDS levels (e.g., 0-2000 ppm) may increase yield but also pose risks.
1. Impact of pH on Gas Production and Energy Consumption
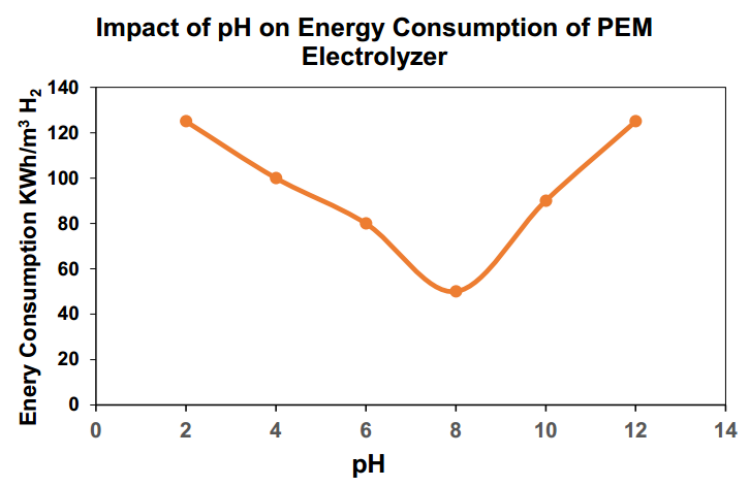
pH is a crucial factor influencing hydrogen production efficiency. Experimental results show that gas production rates fluctuate with pH changes. As pH increased from 3 to 7, gas production decreased, suggesting a neutral environment might slow the electrolysis process. However, when pH rose to 11, production increased significantly, indicating alkaline conditions can favor hydrogen generation.
pH directly affects energy consumption. The optimal pH range for PEM electrolyzers is typically between 7 and 9. pH influences electrolyte conductivity: excessively high pH can damage the membrane structure, increasing energy consumption, while overly low pH reduces conductivity and can cause membrane drying, also raising energy use. Experimental data indicated the lowest energy consumption (approximately 45 kWh/m³ H₂) at pH 8, with deviations increasing energy demand.
2. Impact of Total Dissolved Solids (TDS) on Gas Production and Energy Consumption
TDS measures the total concentration of dissolved inorganic and organic substances and is a key water quality indicator. Tests at low (300 ppm), medium (600 ppm), and high (900 ppm) TDS levels showed that gas production increased with rising TDS concentration. High TDS might act as a catalyst, promoting hydrogen formation. Conversely, low TDS levels resulted in limited production, and zero TDS yielded no hydrogen, consistent with existing research.
TDS significantly impacts energy consumption. High TDS increases water conductivity but can elevate the cell voltage, leading to higher energy consumption. Furthermore, TDS can cause scaling on electrodes or the membrane, reducing efficiency. Implementing water treatment technologies like reverse osmosis or deionization is recommended to reduce TDS and optimize energy usage.
3. Impact of Conductivity on Gas Production
Conductivity, reflecting ionic concentration in water, is another vital parameter. High conductivity can lower the overpotential for the Anode Oxygen Evolution Reaction (OER), reducing energy requirements. However, excessively high conductivity increases the risk of membrane degradation and pumping energy losses. Experiments conducted at 30 mS/cm, 70 mS/cm, and 100 mS/cm confirmed that increased conductivity promotes hydrogen production, aligning with multiple studies.
4. Comparative Impact of Different Water Types on Energy Consumption
A comparison of seawater, well water, and deionized water reveals distinct effects:
Seawater: High dissolved salts and minerals increase conductivity but also elevate resistance, requiring higher operating voltages and thus increasing energy consumption.
Well Water: Generally contains fewer dissolved solids than seawater, leading to lower energy consumption, but variable mineral composition introduces uncertainty.
Deionized Water: Low conductivity minimizes resistance, enhancing energy efficiency. However, the lack of necessary ions requires careful system design considerations for effective operation.
Conclusion and Implications
While PEM water electrolysis research often focuses on the stack itself, the management of the Balance of Plant (BOP), particularly the feedwater system, is equally critical. Optimizing water quality parameters—pH, TDS, and conductivity—can enhance efficiency, increase gas production, and extend equipment lifespan. Although the BOP for PEM systems is simpler than for alkaline systems, strict pure water quality management remains paramount for optimal performance and cost-effectiveness.







Comments